Getting to the roots of acute myeloid leukemia
New insights into the role of the transcription factor HLX in acute myeloid leukemia
Acute Myeloid Leukemia or AML is one of the most studied blood cancers. However, many unsolved questions remain and currently the five-year overall survival rate for AML patients is only 26 percent. Researchers from the Max Planck Institute of Immunobiology and Epigenetics in Freiburg, Germany were now able to define the mechanistic role of a protein, namely HLX, which is overexpressed in many AML cases. Eirini Trompouki and her team proved that HLX regulates metabolic genes and upon its overexpression metabolic deregulation causes differentiation blockade in precursor cells of human blood. With these findings the study also lays new foundation for potential new therapeutic approaches for AML.
Acute myeloid leukemia (AML) is a blood cancer that represents the most common type of acute leukemia in adults. It is often caused by genetic mutations of blood-forming cells of the bone marrow. The malignant cell in AML is a myeloblast, an immature progenitor of white blood cells. In patients, the malignant myeloid cells accumulate genetic changes preventing them from further differentiation and leading them to uncontrolled proliferation.
The current inability to fully treat AML is attributed to its complexity, often stemming from the heterogeneity of individual mutations each patient carries. Identification of all individual mutations for each patient is one of the new era solutions offered to overcome this problem. For this reason, researchers worldwide investigate genes and their function involved in the disease to offer better personalized treatment to patients in the future.
HLX gene involved in most AML cases
Researchers in the laboratory of Eirini Trompouki at the Max Planck Institute of Immunobiology and Epigenetics reasoned that, instead of identifying individual mutations, exploring the precise mechanism of factors involved in many AML cases and, as such, possibly playing a central role in the disease, could offer new therapeutic strategies.
Despite numerous studies on AML, the Trompouki lab members were surprised to find out that there was a transcription factor, namely HLX, previously reported as overexpressed in 87 percent of AML patients, but its precise mechanism of action was largely unknown. Transcription factors are specific proteins that bind to DNA. They are similar to genetic light switches and capable of turning genes on and off. In the case of HLX, a connection with blocking myeloid cell differentiation in mice was observed before, but it remained unclear how exactly the factor works and most importantly what role it plays in healthy or leukemic cells of humans.
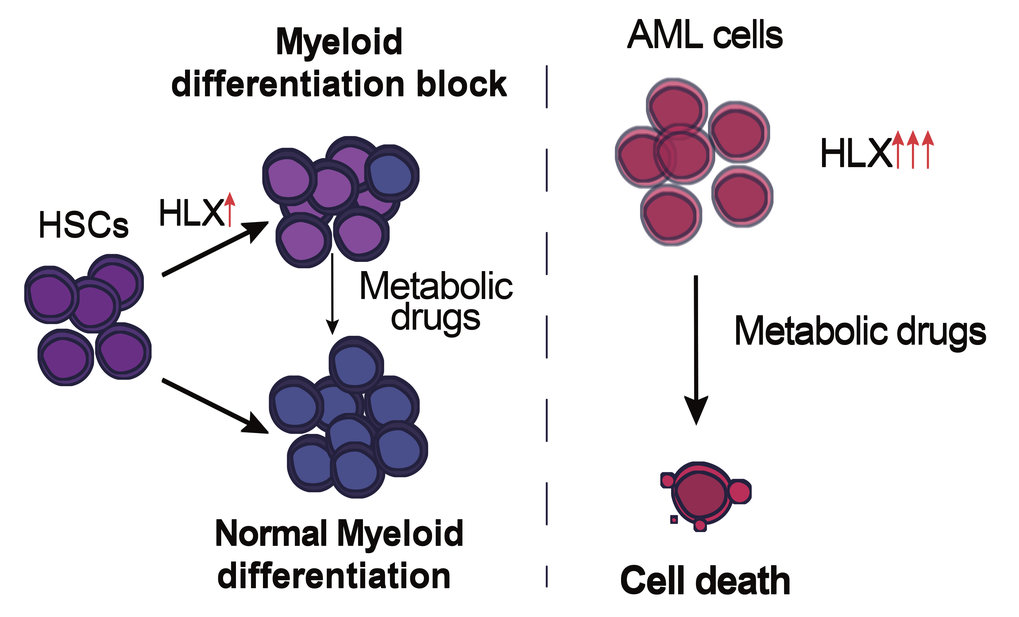
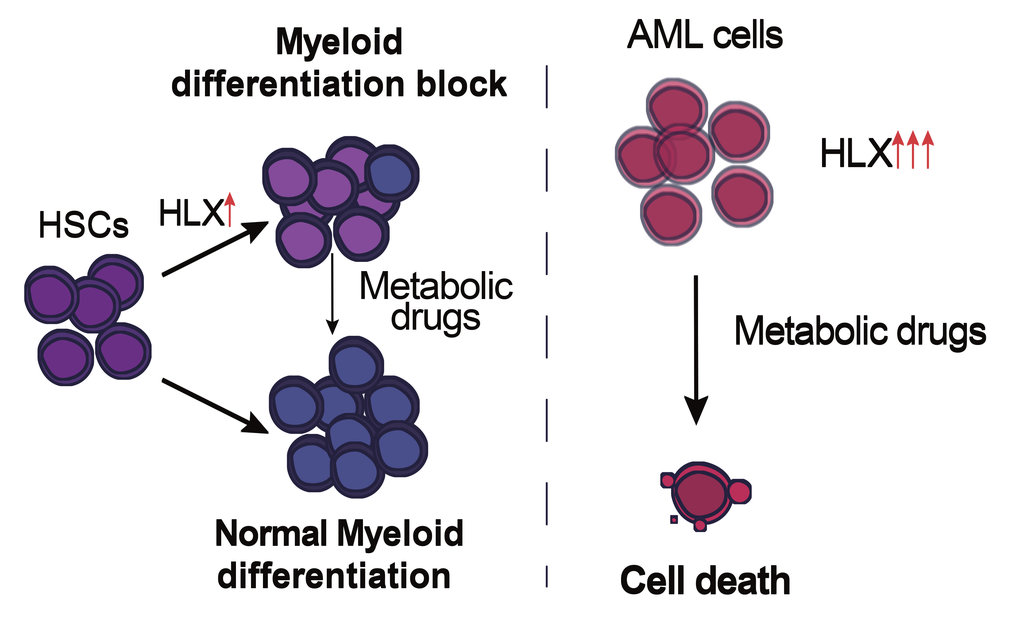
“To overcome the obstacles of the disease complexity caused by the presence of several deregulated pathways and delineate the role of this factor in the hematopoietic system, we decided to first go back to the roots and investigate what HLX is normally there for,” says Eirini Trompouki, who conceived and led the project together with Indre Piragyte, Thomas Clapes and Aikaterini Polyzou as first authors of this study. The scientists used zebrafish embryos and overexpressed the human HLX gene specifically in the embryonic aorta, where HSCs are born, or directly in human hematopoietic stem and progenitor cells (HSPCs). In both cases, there was a block in the differentiation of myeloid cells, consistent with the implication of HLX in a myelogenous type of leukemia.
HLX regulates the energy potential of the cell
As the central question of the authors was how HLX mediates its role, they combined several genome-wide techniques in both zebrafish and different human cell lines. “To understand the role of HLX, we created a panel of genome-wide datasets illuminating the molecular profile of cells where HLX is overexpressed or absent. Metabolic deregulation was a common observation in all our data,” says Aikaterini Polyzou. Her colleague Thomas Clapes adds: “We were also able to perform analyses that show for the first time the genes that are directly regulated by HLX. The results clearly show that HLX affects metabolic genes.” Specifically, the authors detected deregulation of the electron transport chain genes that are located in mitochondria as well as other proteins involved in metabolic regulation, like members of the peroxisome proliferator-activated receptors or the metabolic sensor AMPK, which was identified as a downstream target of the molecular events initiated by HLX.
“The fact that metabolic regulation plays such a central role in the hematopoietic system, both under normal and malignant conditions, was not surprising at all. The metabolic profile of HSPCs or cancerous blood cells is a crucial factor for the fate and function of these cells,” explains Eirini Trompouki. Based on that, the Freiburg researchers tested whether pharmacological modulation of the metabolic pathways regulated by HLX could alleviate the myeloid differentiation block or have an impact on the survival of cancerous cells. Strikingly, the team identified that pharmacological agents acted differently on normal cells or on AML cells. In detail, pharmacological inhibition of AMPK, the metabolic sensor upregulated upon HLX overexpression could cause lethality on cancerous cells while minimally affecting normal cells. “We strongly believe that pharmacological inhibition of AMPK should be further explored as a potential treatment for AML patients, since it severely affects the viability of cancerous cells, but has a milder impact on healthy cells,” concludes Eirini Trompouki.