Chromosome centromeres are inherited epigenetically
The histone protein CenH3 is both necessary and sufficient to trigger the formation of centromeres and pass them on from one generation to the next
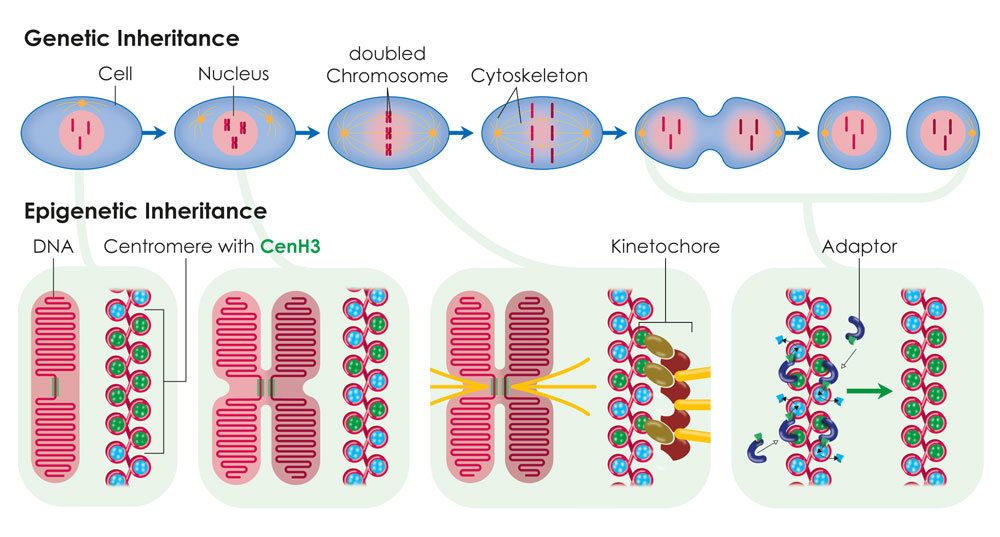
Centromeres are specialised regions of the genome, which can be identified under the microscope as the primary constriction in X-shaped chromosomes. The cell skeleton, which distributes the chromosomes to the two daughter cells during cell division, attaches to the centromeres. In most organisms the position of the centromere is not determined by the DNA sequence. Scientists from the Max Planck Institute of Immunobiology and Epigenetics in Freiburg have succeeded in demonstrating that the position, function and inheritance of the centromere are determined by the histone CenH3, a DNA packaging protein. This discovery may help to further the development of artificial human chromosomes, which could be used for gene therapies in medicine.
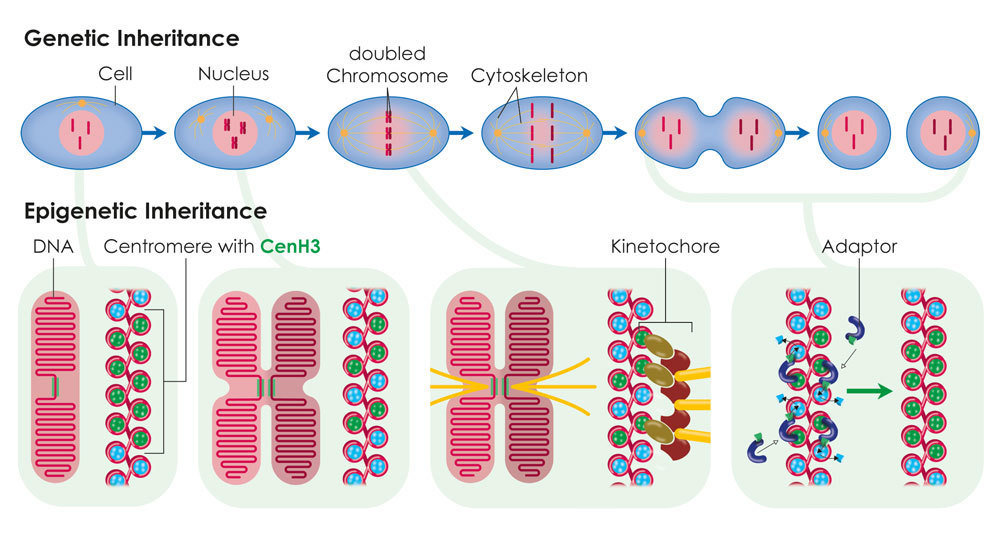
Centromeres provide a platform for the development of a protein complex known as the kinetochore. During cell division, the kinetochore provides a point of attachment for the cell skeleton and enables the chromosomes to move to the opposite poles of the cell. In most organisms the position of the centromere is not determined by the sequence of the DNA building blocks, i.e. the DNA sequence, but epigenetically. The only exception to this rule is the unicellular fungus baker’s yeast, in which a specific DNA sequence “encodes” the position of the centromere.
A particularly promising candidate for this kind of epigenetic centromere marking is a variant form of the H3 histone known as CenH3. Histone proteins bind the DNA largely independently of the underlying sequence and help to package the long thread-like DNA molecule. CenH3 arises exclusively in DNA regions at the centromere in various organisms. Patrick Heun’s research group at the Max Planck Institute of Immunobiology and Epigenetics and colleagues from the Helmholtz Research Center in Munich have now discovered that CenH3 alone is sufficient to trigger the formation of the centromere.
For their experiments, the researchers equipped the CenH3 histone with an artificially attached DNA binding domain so that the protein could bind to a DNA region where a centromere does not normally form. Now however, a functioning kinetochore arose there which interacted with the cell skeleton during cell division. Using this approach, the researchers succeeded in distributing artificial minichromosomes between the two daughter cells during cell division. The protein is able to recruit additional CenH3 proteins independently. “This ensures that sufficient CenH3 is available at the centromere after each cell division. Otherwise, the available CenH3 proteins would be reduced by half after each cell division. In this way, the centromere position can be passed on from generation to generation,” says Heun.
The step from a DNA-identified centromere in baker’s yeast, in which the position “is set in stone”, to a protein-defined centromere position which is easier to change may also play a role in evolution. Despite being up to several million DNA building blocks in size, centromeres can “jump” to other positions without causing the DNA to move. Consequently, in rare cases, a new centromere can arise as it has already occurred in a closely-related ape species. Therefore neo-centromeres might contribute to the emergence of new species.
The insights into the central role of CenH3 for centromere identity could also prove important for medicine. Scientists would like to develop artificial human chromosomes as an alternative to gene therapy using viruses. “Like their natural counterparts, these require a centromere for cell division. Up to now it has not been possible to control the development of a centromere efficiently,” says Heun.
HR