Understanding the molecular lego
Structural insights into the histone-modifying complex NSL and its interaction partners reveal therapeutic danger spots.
In many human diseases including cancer, epigenetic factors such as chromatin-modifying complexes are misregulated. One direction of drug discovery is to target these complexes in the cells. This requires detailed information about the molecular architecture and structure of these chromatin-modifying complexes. Now scientists working together in Max Planck Institute of Immunobiology and Epigenetics (Freiburg) and EMBL (Grenoble) have provided novel insights into a relatively new player in chromatin-modification, the Non-Specific Lethal (NSL) complex. Malfunctions of this complex in humans have been reported to lead to intellectual disability or even to developmental lethality. The findings have also implications for a recently proposed targeted cancer therapy.
Cellular gene expression is highly regulated. One prominent level of regulation is the alteration of the DNA-protein polymer called chromatin. Chromatin changes result in higher or lower accessibility to the expression machinery. The NSL complex is one of those regulators in the fruit fly, controlling more than 4000 genes.
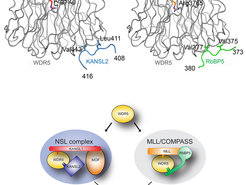
In a joint effort between the labs of Dr. Asifa Akhtar (MPI-Freiburg) and Dr. Jan Kadlec/Dr. Stephen Cusack (EMBL-Grenoble) researchers, for the first time, were able to identify the molecular architecture of the NSL complex and shed light on the role of the protein WDR5 in the assembly and function of the NSL complex. They found that WDR5 uses the same interfaces to interact with the NSL-components KANSL1 and KANSL2 as it does with the proteins MLL(s) and RbBP5 in the well-known MLL complexes. Mis-regulation of the MLL complex often results in the mixed lineage leukemia.
This result has changed the researchers’ view about the protein WDR5. “Until now, it was considered to bridge two different chromatin-modifying complexes, the NSL and MLL complexes. But in fact it accommodates mutually exclusive interactions for integrating and separating signals within individual histone modifying complexes,” said Dr. Akhtar.
They also showed that the interaction of WDR5 with KANSL1 (hence with MLL as well) is very important for the viability of Drosophila fruit flies. Therefore, due to the high conservation of the NSL complex from flies to humans, this interaction might also have crucial roles in humans.
However, this finding has also raised the awareness about potential risks of a promising type of cancer therapy for the mixed lineage leukemia. A recently proposed therapy would use newly identified drug candidates that target the connection between WDR5 and MLL(s). This targeting now needs to be reconsidered because it also affects the interaction between WDR5 and KANSL1, and as a consequence the integrity of the NSL complex.
The NSL complex has been identified in 2006 by the Akhtar laboratory. “It is essential to gain detailed knowledge of how epigenetic regulatory multiprotein complexes are build up “like a lego puzzle”. Only then we will succeed in gaining momentum in designing specific drugs for epigenetic therapy,” said Dr. Kadlec. For their study the researchers combined approaches ranging from structural biology to genetics using mammals and flies.
