Slow walkers
Mast cells deploy adhesion receptors to move inside tissues
Mast cells, known for triggering unwanted allergic reactions, are tissue-resident immune cells that act as sentinels and respond to noxious stimuli by alarming other cells. However, little is known about how mast cells organize themselves in tissues. At the Max Planck Institute of Immunobiology and Epigenetics in Freiburg, researchers have developed genetic mouse models and special tissue-mimicking assays to track the movement strategies of this important cell type. In stark contrast to other immune cells, mast cells critically depend on integrin-mediated adhesion and interactions with extracellular matrix to enable a slow migration and site-specific positioning in tissues. This makes mast cells unique among the broad range of immune cells in our body.
Mast cells have a long history in Freiburg. In the winter of 1876, medical student and later Nobel Laureate Paul Ehrlich discovered mast cells at the Institute of Anatomy in Freiburg. When investigating them under the light microscope, he was struck by the large number of filled membrane bubbles inside the cell. These granules mistakenly led him to believe they were overnourished cells, so he named them “Mastzellen” from the German “mästen” for to feed somebody up.
However, scientists soon realized that mast cells produce these granules themselves to store a plethora of pro-inflammatory substances, which can be released upon contact to different potentially dangerous signals from our environment. This granule release triggers an inflammatory defense reaction, which often involves the swelling of the tissue and the recruitment of other immune cells from our blood. Thus, these long-lived immune cells are part of important defense mechanisms, such as the degradation of venom toxins. In many people, mast cells also react to seemingly harmless environmental factors, which then act as allergens. This also leads to the release of the same pro-inflammatory substances and the triggering of allergic or anaphylactic reactions, which mast cells are probably best known for.
Mast cell migration in tissue
Today, advances in imaging technologies and molecular biology make it possible to study mast cells at a whole new level. A research group at the Max Planck Institute of Immunobiology and Epigenetics in Freiburg has the expertise to track the dynamic behavior of immune cells in 3D tissue assays and native mouse tissue. The team led by Tim Lämmermann now reveals a more nuanced understanding of how mast cells migrate, position and organize themselves in tissues.
Their findings, published in the journal Nature Immunology, show that mast cells critically depend on integrin receptors and adhesive interactions with the extracellular matrix (ECM) to generate enough forces for their movement in tissues.
Cell movement: fast squeeze or slow pull?
“Although it has been known for almost 30 years that mast cells express and use integrin receptors, it has remained unanswered whether and how integrin receptors and their interactions with the ECM contribute to mast cell organization and movement in tissue,” says Tim Lämmermann.
Most immune cells migrate in a fast amoeboid fashion and do not require strong adhesive interactions with their surrounding when moving through interstitial tissue spaces. They move autonomously and independently of the biochemical composition of the tissue environment simply using cytoskeletal forces to change shape and generate propulsion.
“In contrast, our observations show that mast cell movement differs fundamentally from the prevailing paradigm how immune cells move,” says Lukas Kaltenbach, one of the first-authors of the publication.
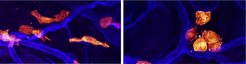
The combined results from Lukas Kaltenbach, Paloma Martzloff and Sarah Bambach, three PhD students in the Lämmermann group, convincingly show that mast cells use integrin-mediated cell adhesion to the extracellular matrix for effective force application. The cells grow protrusions that appear like temporary limbs. The integrin adhesion receptors on the cell’s surfaces serve as sticking points, enabling them to maintain contact with the surrounding material. “Mast cells then pull themselves forward while simultaneously releasing adhesions in their rear part to allow productive movement. While this has been known for many other non-immune cells, this is new for the immune cell world” explains Tim Lämmermann.
Mast cell networks in tissues
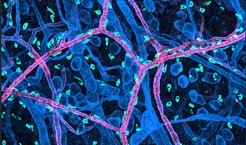
Their movement strategy makes them unique among immune cells and appears perfectly adapted to their role as tissue-resident cells. Mast cells act as sentinels, responding to noxious stimuli by attracting additional immune cells and triggering an inflammatory response. The slow integrin-mediated interstitial movement is crucial for organizing mast cell distribution in physiological tissues. Mast cell populations are found primarily in tissues in direct contact with the outside world, i.e., especially in the skin or mucous membranes of the respiratory tract or intestine.
“Our data also suggest a special role of integrins for positioning MCs around arterioles. These areas around this special blood vessel type are rich in mast cell-specific growth and survival factors, which helps to establish long-living mast cell networks. This is an interesting biological aspect that we will follow up in future studies” says Paloma Martzloff, another first-author of the study.
The findings reshape our view on tissue-resident immune cell migration and let the scientists consider mast cells as potentially mechanosensitive immune cells. This could inform future studies on mast cell responses to changing tissue properties under various healthy and diseased conditions, including mast cell-dependent inflammatory conditions or mastocytosis.
TL/MR













