Mapping RNA signatures in neurons
Max Planck researchers show the precise temporal protein orchestration for correct brain development
The ELAV/Hu protein family is one of the most important group of proteins in neurons. Every neuron of every animal expresses at least one ELAV/Hu protein. If ELAV/Hu proteins are absent, the nervous system cannot develop and animals die as embryos. The lab of Valérie Hilgers at the Max Planck Institute for Immunobiology and Epigenetics in Freiburg identified the key players and the mechanism behind ELAV’s function in brain development. By creating a series fruit fly mutants using CRISPR/Cas9 genome editing, they draw a genetic map connecting ELAV/Hu activity at different stages of neurogenesis, to the gene targets, and the physiological outcome.
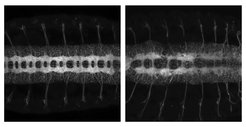
During embryonic development each neuron undergoes drastic changes in shape and function. The transformation allows them to become fully functional and establish connections with other neurons. A well-regulated activity of various molecules is necessary for a successful transition and essential for correct neuronal development and, ultimately, for brain function. The laboratory of Valérie Hilgers at the MPI of Immunobiology & Epigenetics studies the molecular effectors and mechanisms that orchestrate these processes.
Just-in-time activity of ELAV
“We already knew that the ELAV protein is key for neuronal development in nearly every animal – from the simplest organism to humans. Our former studies showed that this is because ELAV mediates the formation of neuron specific RNA signatures. And without the protein, neurons lose their cellular identity and molecularly resemble non-neuronal cells. However, how and when ELAV acts or should act is still a mystery,” says Judit Carrasco, the study’s first author.
The team studied the neuronal development in the fruit fly Drosophila melanogaster. They generated fly mutants using CRISPR genome editing to switch ELAV expression on and off at different stages of their development. “We found that there is a brief time window at the onset of neuronal differentiation, in which ELAV is absolutely critical for life. If the protein is not active at this point in time, the flies cannot survive. Later on, neurons need ELAV to function properly, and reduction of the expressed protein levels causes severe neurological phenotypes,” says Max Planck research group leader Valérie Hilgers.
Critical ELAV function at the onset of neuronal differentiation
The importance of precise temporal regulation of ELAV expression and its consequences in the course of neuronal development is because ELAV is a master regulator of the RNA biology in neurons. “Messenger RNAs are intermediates of gene expression between DNA and protein. Interestingly, most genes can produce several variants, or isoforms, of messenger RNAs,” explains Judit Carrasco. The production of these alternative RNA isoforms is mediated by the ELAV protein and plays a central role in the diversification of the cellular transcriptome, giving each cell type a unique molecular signature.
By using deep sequencing technologies, the authors could show how critical the activity of ELAV is at the onset of neuronal differentiation. In contrast to the hundreds of RNA isoforms affected in later developmental stages, the loss of just a few dozen RNAs at the beginning of differentiation causes irremediable consequences for life. Interestingly, most of the gene targets are affected in their regulatory regions known to determine the fate of the RNA in the cell. The scientists suggest that this creates new opportunities to investigate the role of post-transcriptional regulation of RNAs in neuronal differentiation.
A rescue mechanism compensates for fluctuations in ELAV levels
In humans but also other organisms, defects in ELAV proteins have been associated with multiple neurological conditions. It is not surprising that complex organisms have developed back-up mechanisms to safeguard critical cellular processes maintained by the protein. In previous work, the Hilgers lab already discovered the EXon-Activated Rescue (EXAR) mechanism that steps in if ELAV is absent. Then FNE, a gene of the same family, changes its subcellular localization to go to the nucleus and take over ELAV functions.
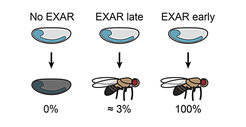
In this study, the team investigated conditions in which EXAR could be a lifesaver or beneficial for animal fitness. “We found that EXAR is a very effective mechanism that compensates for extreme variations of ELAV levels – up to 95% loss. “Minute amounts of ELAV activity at the onset of differentiation are sufficient to overcome developmental defects and, later, EXAR can fully rescue all ELAV functions” says Valérie Hilgers. The team engineered a fly model with very early and high EXAR activation and showed that in these conditions, it could compensate for all ELAV functions, even in the critical developmental window. “In terms of neuronal function and global physiological fitness, these flies are indistinguishable from wild type with correct ELAV levels” adds Hilgers.
This study sheds light on the functional consequences of the loss of ELAV function at different points of development and characterizes the internal rescue mechanism to safeguard cell type identities by interaction between the members of the ELAV protein family. Looking forward, the Hilgers lab plans to investigate how defects in ELAV expression in response to aging and other environmental signals plays a role in neurodegeneration.
JC/VH/MR













