Maintaining the brain’s maturity
Researchers identify neuronal granules as guards of the adult nervous system
Neurons in fruit flies and other organisms use membrane-less cellular compartments consisting of RNA molecules and proteins to regulate their gene expression and cellular processes. Researchers at the MPI for Immunobiology and Epigenetics have now discovered how and when these ribonucleoprotein (RNP) granules form and were able to show that a specific type of RNP granules is crucial for maintaining the maturity of the adult nervous system. These findings in the fruit fly Drosophila melanogaster also have relevance for research into neurodegenerative diseases.
The nervous system of any organism is predominantly made up of neurons. During development, neural stem cell divide into neurons, which progressively establish an intricate network of connections. Neurons communicate through this network by sending and receiving electrical signals.
The precise regulation of gene expression is critical for the functioning of neurons in the developing and mature brain, much of which occurs through changing levels or locations of the messenger molecule RNA. Neurons employ various strategies to ensure the robustness of their RNA regulation, including the formation of ribonucleoprotein (RNP) granules. These are membrane-less cellular compartments composed of RNA and RNA-binding proteins that tune time and location of protein expression and affect many cellular processes that require complex protein distribution.
lncRNA mimi as determinant of protein condensation in neurons
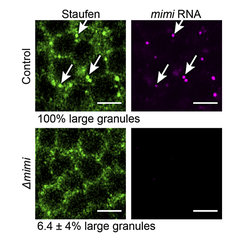
Neurons assemble proteins and their associated RNAs into these granules to optimize efficiency and coordinately regulate large sets of transcripts. Interestingly, the deletion of key granule proteins not only causes the disassembly of the granule, but also disrupts other protein functions. “Until now, it was not possible to uncouple the role of neuronal granules per se, from the function of granule-constituent proteins”, says Valérie Hilgers, Group leader at MPI of Immunobiology and Epigenetics. However, using Drosophila melanogaster as a research model, the lab of Valérie Hilgers identified a highly specific and absolutely necessary component of a particularly large and widespread neuronal granule type that can also be found in abundance in the brain of many other animals including humans.
The team named this component mimi, after its discoverer and doctoral researcher in the Hilgers lab, Dominika Grzejda. “When we removed mimi, the formation of the granules was disrupted, but not the expression of major granule-forming proteins,” says Dominika Grzejda, first author of the study.
mimi granules maintain nervous system maturity
Studying neurons of the flies, the researchers were able to determine the physiological function of the mimi granule. It turned out that mimi RNA is a very unusual type of RNA: it does not, like messenger RNAs, encode a protein. It is completely absent from the brain during development and only starts to be expressed when flies enter adulthood – a stage that in Drosophila, begins with the eclosion from the pupae. Once expressed, mimi is extremely abundant. However, it can be only found in one particular place: granules. As a consequence, mimi granules start assembling around mimi once the brain is fully developed. The scientists in Freiburg show that mimi acts as an architectural RNA for the neuronal granule and provides for the first time a handle to interrogate a granule’s functions independently of its constituent proteins.
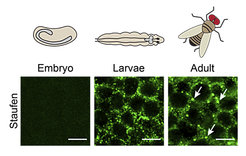
“We found that mimi granules are essential to perform adult neuronal functions and to maintain the neuron in a mature molecular state”, says Dominika Grzejda. Loss of mimi granules disrupts neuropeptide-mediated signaling and upregulates cell cycle genes, which are normally repressed in non-dividing, terminally differentiated adult neurons. Interestingly, the loss of mature neuronal transcriptome pattern in mimi mutant flies causes phenotypes of neurodegeneration. The scientists observe progressive deterioration of motor functions and a shorter life.
The study by the Hilgers lab advances our understanding of the mechanisms associated with the condensation of neuronal RBPs and provides insights into in vivo consequences of granules hypo-assembly. This brings us one step closer to developing effective treatments for neurological diseases resulting from the dysregulation of RNA-protein condensation.
DG/VH/MR

