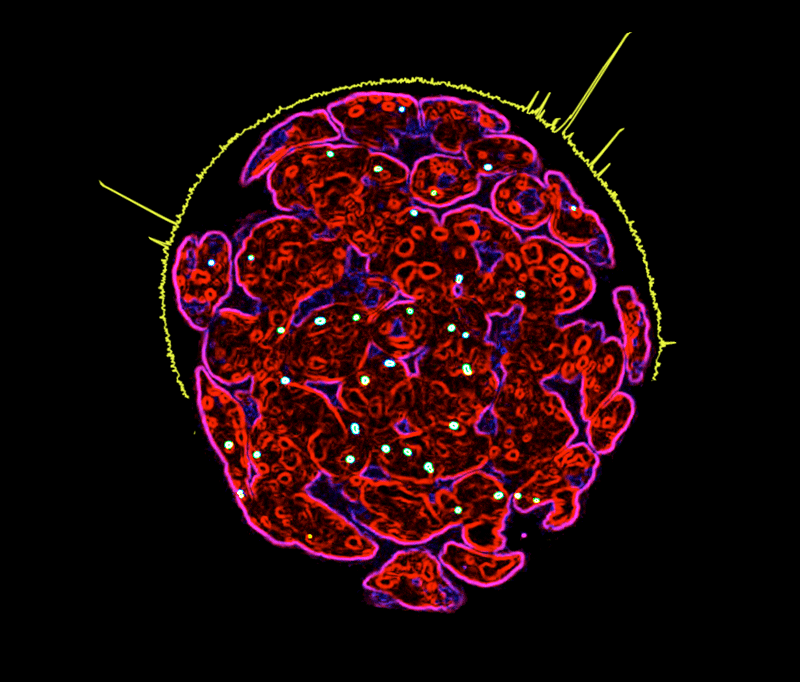
The Battle of Xs
MOF complexes mediate genetic fair play
Sexually dimorphic animals are often distinguished by unequal number of the X-chromosomes. While males have only one X chromosome, females have two copies, prompting an evolutionary pressure for compensatory mechanisms against this disequilibrium. Some species, such as fruit flies, up-regulate the single X chromosome in males, while other species, such as mouse and human, silence one of the two X in females. Now scientists from the Max Planck Institute of Immunobiology and Epigenetics in Freiburg have discovered that the same, evolutionary stable MOF protein that regulates X dosis in flies is also involved in compensatory mechanisms in mice. Interestingly, MOF-mediated regulation of X-inactivation is achieved by parallel action of not one, but two distinct complexes.
In male fruit flies the protein complex called MSL together with its major enzyme called MOF pushes the single X chromosome to express its genes with double efficiency. Mice also struggle with different number of X’s between the sexes, but in contrast to flies, where ‘X up-regulation’ takes place, here the females inactivate one of their two X chromosomes in process called ‘X inactivation’.
The team, led by Asifa Akhtar, Director at the Max Planck Institute of Immunobiology and Epigenetics in Freiburg demonstrated that two – throughout the evolution extremely conserved – protein complexes have impact on upregulation and inactivation. Both complexes orchestrate the function of the gene regulator MOF. “What we find most intriguing is: MOF and its protein partners maintain the activity of both X chromosomes in female stem cells, which is essential for preserving their unique charcter,” said Akhtar. “It was overwhelming for us to see that the same protein engages in the specific regulation of X chromosomal dose both in flies as well as mouse, where these mechanisms seem world apart,” continued the co-lead author Tomasz Chelmicki. In addition, the MOF-associated complexes influence the expression of thousands of genes in mouse cells.
During development of female mammals one of the two X chromosomes has to be inactivated in order to achieve the identical number of genes in male and female individuals, a process called ‘dosage compensation’. However, in embryonic stem cells both X chromosomes have to remain active. The study now shows that the MOF protein complex plays a central role in this X chromosome regulation. The MOF-MSL complex regulates the gene Tsix, which inhibits the production of Xist – an RNA molecule responsible for X chromosome inactivation. The protein complex MOF-NSL ensures the preservation of stem cell identity through activation of several factors and thus effectively antagonizing the expression of the RNA Xist, that would ultimately lead to X-inactivation.
Detailed insights into genome-wide interactions of MSL and NSL were possible due to a combination of powerful sequencing techniques and biochemical experiments. “Analyzing the continously growing amount of data that we obtained with high-throughput methods is a real challenge,” said co-lead author Friederike Dündar. “But it also offers the opportunity to study how different complexes can cooperate and complement each other in order to reach the same goal in the cell.”
The MOF enzyme is responsible for histone acetylation. This posttranslational modification results in a better accessibility of the expression machinery to the DNA. The knowledge gained form this study will pave the way towards better understanding of complex processes such as embryonic development, organogenesis and pathogenesis of disorders like cancer.
JF/HR