Janus-faced MOF
When Epigenetics meets Metabolism
New research from Max Planck Institute of Immunobiology and Epigenetics, Freiburg (MPI-IE) sheds light on gene regulation in the nucleus and mitochondria. Published in Cell, the study by the lab of Asifa Akhtar show that a classical epigenetic regulator MOF is a dual transcriptional regulator acting on nuclear and mitochondrial genomes in our cells. Thus, MOF appears to be a molecular bridge-builder connecting epigenetics and metabolism.
Asifa Akhtar – How Does the Enzyme MOF Work as a Molecular Bridge between Epigenetics and Metabolism?

DNA encoding our genetic information is not naked in the cells, but is instead packaged by proteins known as histones in the nucleus. MOF is a well-known enzyme that resides in the nucleus and modifies histone proteins so that the information coded in the DNA can be read more easily. MOF controls “housekeeping genes” which like the name suggests are essential for the day to day running of the household thereby the “existence” of the cell, encoding proteins that are indispensable for functions such as energy metabolism, cell division, or DNA repair.
Interestingly, the nucleus is not the only site in cells storing DNA. Also mitochondria that are widely recognized as the powerhouse of the cell have their own circular DNA, distinct from nuclear DNA in origin and resembling bacterial genomic DNA. In cooperation with nucleus, the few genes of the mitochondrial DNA are essential for cellular energy production. However, how exactly these two organelles coordinate their gene expression remains largely unknown.
Now, researchers at the MPI-IE in Freiburg unravel a new facet of the enzyme MOF. It turns out that MOF not only sweeps the broom by controlling “housekeeping genes” of the nucleus DNA, but also has key role as gene regulator in mitochondria DNA governing important cell functions like the cellular energy production.
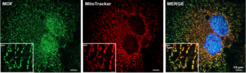
Figure 2 Fluorescence microscopy images of human (HeLa) cells expressing green fluorescent proteins tagged MOF (green) co-stained with red fluorescent dye for mitochondria (red) and DAPI for nucleus (blue).
Using confocal microscopy, biochemistry and genetics, the team led by Asifa Akhtar was able to detect a significant pool of MOF in the mitochondria (Figure 2). Here, MOF controls expression of genes, which regulate oxidative phosphorylation (OXPHOS). As part of cellular respiration, OXPHOS is one of the core metabolic pathways for generating energy.
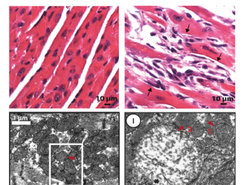
Figure 3 Electron microscopy images of cardiac muscle (cardiomyocytes) tissue from normal and Mof deleted mice are shown. The upper panels show the tissue structure. The lower panels give a more detailed view of the mitochondria. Red arrow 1 indicate a normal mitochondria. Red arrow 2 indicate a degenerated and dysfunctional mitochondria.
The importance of MOF for mitochondrial function was shown by several experiments but a very dramatic visualization can be seen in experiments performed with heart muscle cells. This cell type is typically rich in mitochondria due to its high-energy requirements. Following MOF depletion, the team noted severe cardiac fibrosis. “This could be actually seen not only by the sudden cardiac arrest in mice but also under the microscope. In cells with Mof loss, we found that typical structure of muscle cells have dramatically changed. We also saw numerous degenerated mitochondria within these cells,” says Aindrila Chatterjee, first author of the study (Figure 3).
The scientists propose that MOF may serve a sensory function to coordinate gene expression in both nucleus and mitochondria, based on the cellular metabolic status. “Finding this classical epigenetic regulator in both compartments of the cell is remarkable. MOF’s involvement in mitochondria paves the way to understand the crosstalk between epigenetics and metabolism. Since MOF is dysregulated in several diseases such as cancer, these findings provide an exciting new perspective to study problems arising in healthy versus diseased state” says Asifa Akhtar.














