Trapped on roX
RNA stem-loop is crucial for the survival of male flies
In many species the sexes have different sets of chromosomes. In humans as well as in the fruit fly Drosophila females have two X chromosomes, while males have one X and one copy of the much smaller Y chromosome. The latter carries very few genes, while the X chromosome has thousands of genes. Male fruit flies compensate for this short-coming by doubling the activity of their single X chromosome in a process called “dosage compensation”. If male flies fail to compensate, they die!
Dosage compensation is mediated by a special set of proteins (MSL complex) and non-coding RNAs. The lab of Asifa Akhtar at the Max Planck Institute of Immunobiology and Epigenetics (MPI-IE) is studying dosage compensation in flies by investigating the interactions between involved proteins and RNAs. In their newest study the team focused on the protein MLE that unwinds RNA structures and mainly works on two large non-coding RNAs called roX1 and roX2.
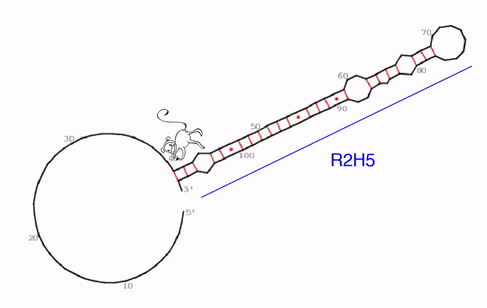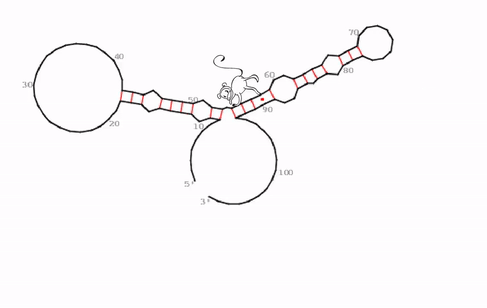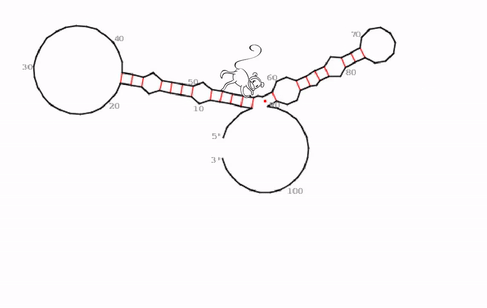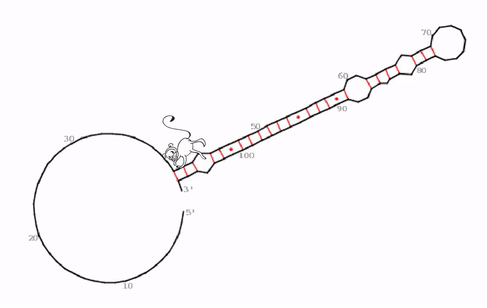
“We identified a very specific, evolutionarily conserved and somehow strange RNA structure that seems to be the functional core of roX2,” says Ibrahim Ilik, first-author of the study. What the team discovered were hitherto unknown stem-loops. In general, stem-loops or hairpin loops are a very common type of secondary structure in RNA molecules that is created when an RNA strand folds and forms base pairs with another section of the same strand. “What makes this stem-loop arrangement so strange, is, that MLE can be trapped in this structure. It tries to unwind the RNA molecule, just to find that, as it unwinds one part, another part forms a loop simultaneously,” explains Ibrahim Ilik.
“We think that the function of this trap is not to just keep MLE endlessly working, but rather to transiently expose a small piece of RNA so that it can interact with other proteins. As MLE goes back and forth, it opens and closes this binding site,” says Asifa Akhtar. As roX2 is remodeled by MLE it enables reversible interactions of MSL proteins to allow dosage compensation of the X chromosome. The team proposes that this ability to open and close a binding site continuously is crucial for the survival of the male flies.
