Help for stressed immune cells
B cells suffer high stress levels at certain stages. The protein MZB1 helps by facilitating the antibody folding.
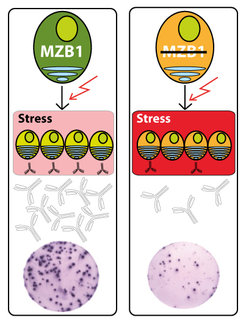
Without the B cells of the immune system, we would be very susceptible to disease. B cells are activated by contact with foreign substances, and mature into high-performance cellular factories called plasma cells, producing up to 2000 antibodies per second. Antibodies are perfectly tailored for fighting off specific pathogenic invaders. However, the conversion of a resting B cell into a plasma cell, and the high amounts of antibodies they produce, causes stress in the cell. Scientists working with Prof. Dr. Rudolf Grosschedl, Director at the Max Planck Institute of Immunobiology and Epigenetics (MPI-IE), have now shown that the protein MZB1 is important to counteract stress during the immune response. When the researchers switched the protein off in mouse B cells, the general immune response was weaker: overall, fewer B cells produced antibodies and in lower quantities. “We knew that MZB1 is important for B cells but we didn’t know its precise function,” explained leading author Dr. Grosschedl. “This has now changed, thanks to the current study.”
To test whether MZB1 is also needed in other stress situations, the researchers investigated immature B cells. To do this, they removed MZB1 in precursor B cells that already suffer from an increased level of stress and subjected them to additional stress. The cells were then unable to develop correctly and showed signs of cell death. It seems the B cells were blocked because they were unable to assemble the precursor B cell receptor, which is very similar to the antibodies produced later in plasma cells. “Our results show that MZB1 plays an important role, both in the efficient formation of antibodies as well as in other stress situations to which the B cell is exposed,” said Dr. Grosschedl, who is head of the Department of Cellular and Molecular Immunology at the MPI-IE.
The MZB1 protein fulfils its function in conjunction with a chaperone. Proteins are synthesized by the cell as a chain of amino acids. Chaperones support proteins during their folding into a three-dimensional structure. “The interaction between the chaperone, MZB1 and antibodies is crucial only under stress,” said Dr. Marc Rosenbaum, first author of the study. “MZB1 brings the chaperone and the antibody together in a very efficient way.” Exactly how this happens is something the researchers would like to find out next.
In some types of cancer, for example chronic lymphocytic leukaemia, MZB1 is present at very high levels and this is often associated with a particularly aggressive course of the disease. MZB1 is therefore being discussed as a target of future therapies. The knowledge gained by the Freiburg-based researchers could contribute to these efforts by providing a more detailed explanation of the role played by MZB1.
