Principles of self-organization
in swarming neutrophils
Lämmermann Lab
Project

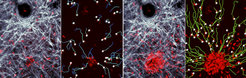
Neutrophils are indispensable effector cells of our innate immune response and regulators of adaptive immunity. As classic phagocytic cells, they engulf pathogens, release lytic enzymes from their granules, produce reactive oxygen species and are hence pivotal for clearing bacterial and fungal infections. Not only are neutrophils key cells for protecting the host from microbial invasion, but they also act as critical mediators of sterile inflammation in acute and chronic diseases. Upon local inflammation or infection, neutrophils undergo phases of highly directed and coordinated migration, followed by neutrophil accumulation at sites of tissue injury or infection, a process termed neutrophil „swarming“. Neutrophils have evolved as true sentinel cells for detecting sites of tissue damage, but the molecular guidance signals that control neutrophil swarming in vivo have long remained unclear.
By using intravital two-photon microscopy, we could define a multistep attraction cascade that guides neutrophils at sites of local sterile injury and identified key molecules controlling individual phases of the swarming response. One of our major findings revealed a critical role for intercellular communication among neutrophils mediated by the lipid leukotriene B4 (LTB4) of the swarming response, which acutely amplified local cell death events to enhance the radius of neutrophil recruitment within the tissue (Lämmermann et al., 2013).
Our previous work provided an initial molecular map for neutrophil swarm formation and novel insight into how neutrophils self-amplify their swarming response (Kienle & Lämmermann, 2016; Lämmermann, 2016). Currently, we are investigating the mechanisms that terminate this response during the resolution phase of inflammation. Ongoing work also explores the functional role of neutrophil swarms in the context of anaphylactic reactions. In a CZI-funded collaborative project with the lab of Eduardo Réategui (University of Ohio) we combine in-vivo imaging and lab-on-a-chip techniques that promise unprecedented levels of detail of neutrophil swarming at the cellular and molecular level.