
Research
Lab Cabezas-Wallscheid
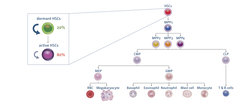
Figure 1 The murine hematopoietic system: HSC = Hematopoietic Stem Cell; MPP = Multipotent Progenitor; CMP = Common Myeloid Progenitor; CLP = Common Lymphoid Progenitor; MEP = Megakaryocyte Erythroid Progenitor; GMP = Granulocyte Macrophage Progenitor; RBC = Red Blood Cell.
Adult hematopoiesis is responsible for the production of billions of mature blood cells every day (Figure 1). It is a hierarchically organized process that almost exclusively occurs in the red bone marrow. Hematopoiesis is tightly regulated and rapidly reacts to stress stimuli, for example blood loss and inflammation, by modulating lineage commitment and terminal differentiation of progenitor cells.
Hematopoietic Stem Cells
Hematopoietic Stem Cells (HSCs) reside at the top of this hierarchy and represent an extremely rare cell population within the bone marrow. HSCs harbor long-term reconstitution capacities and have the ability to generate multipotent progenitors, which in turn differentiate into lineage-committed populations and subsequently into mature blood cells. Another fundamental feature of HSCs is their quiescent cellular status in terms of cell cycle activity and low biosynthetic activity. Quiescence or dormancy preserves and governs the life-long functionality of HSCs and protects them from accumulating genomic mutations potentially acquired during rapid cell divisions.
Regulation of Hematopoietic Stem Cells

Figure 2 Identification of regulatory networks in HSCs and their immediate progeny via integrated proteome, transcriptome, and DNA methylome analysis. Main findings summarizing Cabezas-Wallscheid et al. in Cell Stem Cell 2014.
Regulators of the dormant HSC state include cell-intrinsic signaling pathways as well as soluble components produced by the bone marrow niche. For instance, stress-signals such as interferons, lipopolysaccharide or stress-conditions including chemotherapy are known to cause HSC proliferation, thereby altering their homeostatic dormant status. In contrast to the factors that can activate and promote HSC exit from dormancy, little is known about the mechanism maintaining HSC quiescence. Importantly, dysregulation of this fine-tuned system may lead to aberrant hematopoiesis such as leukemia. Currently, the preservation of the dormant HSC status and the development towards a leukemic stem cell population is not well understood.
We have recently performed an extensive multi-layered OMICs analysis of HSCs and four progenitor populations (MPP1-MPP4) by combining DNA-methylome, whole-transcriptome and global proteome analyses (Figure 2). Our work identified exclusive gene expression clusters as potential gatekeepers of HSC self-renewal such as splicing variants, long non-coding RNAs and retinoic acid metabolism.
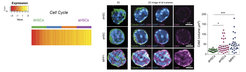
In a follow up study, we have shown by single-cell RNA-seq data that the molecular transition from the most inactive dHSCs cluster to the most active HSCs can be best described as a continuous stream-like process of steadily increasing metabolic activity. First protein synthesis and subsequently cell cycle related components are continuously increasing in cells exiting the dormant state and moving towards an aHSC quiescence and cell cycle primed stage (Figure 3).
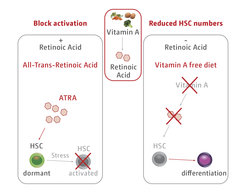
The association of delayed cell cycle entry with the extremely low biosynthetic activity defines the status of dormancy and distinguishes it from quiescence. Furthermore, we have observed that particular dietary habits, mainly vitamin A deficiency, is important for HSC maintenance, findings which pave the way to new fundamental and yet unsolved questions (Figure 4).
The goal of our laboratory is to cover novel ground on mechanisms that maintain HSC quiescence. We aim to investigate the impact of different nutritional regimes on HSC maintenance and to analyze the underlying regulatory mechanisms. Our ultimate goal is to translate these findings into human disease settings such as dietary deficiencies and leukemia. To address these biological questions, we are pursuing interdisciplinary projects which include the use of genetic models, primary patient material in combination with state-of-the-art population and single-cell OMICs analysis.



