Stem cells: you are what you eat
Freiburg stem cell research shows how a vitamin A waste product regulates cell functions in blood formation
Blood stem cells residing in the bone marrow ensure that our blood system is supplied with fresh blood cells – especially in emergency situations such as infections or inflammation. Outside of these emergency situations, blood stem cells remain in a state of dormancy to protect their unique potential. The lab of Nina Cabezas-Wallscheid at the Max Planck Institute of Immunobiology and Epigenetics in Freiburg is investigating the complex regulatory networks that preserve these exceptional capabilities. The team has now revealed how a unique metabolite produced from vitamin A, previously considered a junk by-product, regulates blood stem cell function.
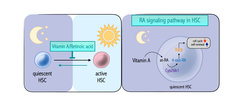
Hematopoietic stem cells (HSCs) sit at the top of the hematopoietic hierarchy and are quiescent. Different stress stimuli, for example infections, can activate this rare cell type. In order to maintain their function, HSCs depend on biochemical signals such as vitamin A/ retinoid acid (RA). HSCs rely on Cyp26b1, an enzyme conventionally considered to limit the RA effects in the cell. In contrast to the traditional view, Cyp26b1 is indispensable for production of the active metabolite 4-oxo-RA. Furthermore, the RA receptor beta (Rarb) is required for complete transmission of 4-oxo-RA-mediated signaling to maintain stem cells.
Blood stem cells, also known as hematopoietic stem cells (HSCs), are a fascinating cell type, making up the foundation of our hematopoietic system. Residing mainly in the bone marrow, they are the only cells that are capable of long-term replenishment of the entire blood system. Every time HSCs are activated and divide, they lose potency and may possibly acquire mutations that could result in leukemic transformation. For this reason, HSCs usually remain in a state of very deep quiescence in which they do not divide and have a very low energy demand. However, in emergency situations such as viral infections or severe blood loss, HSCs give rise to all kinds of blood cells, including immune system cells such as leukocytes.
Metabolism influences cell fate and function
Nina Cabezas-Wallscheid and her team at the Max Planck Institute of Immunobiology and Epigenetics are interested in the fate of HSCs. They aim to understand how these distinct fundamental stem cell functions are regulated and maintained. “One approach our lab follows is to look into the metabolism of this rare cell type. During the last few years, it has become more and more evident that intermediate and end products of metabolic processes play an active role in regulating cell fate,” says Nina Cabezas-Wallscheid.
Cellular metabolism converts nutrients into energy and essential biomolecules which are key to regulating cell functions and consequently cell fate. Different metabolic signatures allow cells, including stem cells, to fulfil their functions in different microenvironments. It has been shown that vitamins in particular regulate HSC function. “Vitamins can act as co-factors of enzymes that regulate the activity of the DNA. Other studies have shown that vitamins suppress leukemogenesis by changing the DNA methylation status in HSCs,” says Katharina Schönberger, co-first author of the study. But working with these small and often transient metabolites in HSCs is extremely difficult. “The low frequency of blood stem cells, just a few thousand per organism, leads to technical challenges. We have put quite a lot of effort into establishing protocols and integrating state-of-the-art low-input omics data for an extensive investigation of the interplay between stem cell metabolism, transcription and epigenetics”, says co-first author Nadine Obier of the Cabezas-Wallscheid lab.
A “junk” metabolite of vitamin A regulates HSC function
One of the most highly enriched metabolic hubs observed by the team was vitamin A/retinoic acid (RA) metabolism. Importantly, human vitamin A deficiency is associated with immunodeficiency and is prevalent among children in developing countries. Current therapies are based on supplementation with vitamin A and are effective in only about 20% of cases. Interestingly, the group found that keeping mice on a vitamin A-free diet led to a profound loss of HSCs and their metabolic identity. To better understand the mechanism of vitamin A signaling in HSCs, the team performed extensive in vitro and in vivo functional knockout studies. As a result, the team uncovered a non-classical RA signaling axis that regulates HSC function. They showed that HSCs rely on an enzyme named Cyp26b1, which is conventionally thought to limit the effects of RA in cells by catalysing the conversion of bioactive at-RA to 4-oxo-RA. In contrast to the traditional view, they demonstrated that Cyp26b1 is indispensable for production of the active metabolite 4-oxo-RA, which mainly transmits signals via the RA receptor beta (Rarb). Overall, the team’s findings emphasize how a single metabolite can control stem cell fate by instructing epigenetic and transcriptional attributes.
NCW/MR





