Indispensable transporter of T cell biology
How a transport protein ensures maturation of signature mRNA of T-killer cells
To support an effective immune defense, T cells depend on the expression of antigen-specific receptors which sense potential pathogens and jump-start the life-saving response. In a recent study, scientists at the Max Planck Institute of Immunobiology and Epigenetics discovered a new mechanism that regulates one of the first steps in the cascade of events that ensure the proper generation of such antigen receptors. They discovered a nuclear import protein without which the production of the mRNA template for a specific receptor component of invariant natural killer T (iNKT) cannot be made. As a result, the typical rapid immune response of iNKT cells is completely abolished, eliminating an important pillar of first-line immune defense.
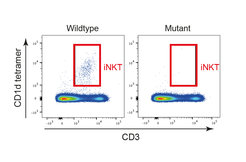
In the vertebrate immune system, conventional helper and killer T cells provide the host with specific immune response capabilities, including the provision of effective memory responses. These conventional T cells express a vast repertoire of structurally diverse antigen receptors known as T cell receptors (TCRs) and are activated by peptide antigen presented by the highly polymorphic classical MHC class I and class II molecules. By contrast, other thymus-derived populations of T cells, such as invariant natural killer T cells (iNKT) exhibit a much more restricted diversity of their antigen receptors, and recognize endogenous and exogenous glycolipids presented by non-polymorphic non-classical MHC-like molecules. These unconventional T cells, also known as innate T cells, do not require prior antigen experience to be able to rapidly respond to the recognition of their cognate antigens. They are thus essential for providing the host with first-line defense capabilities.
iNKT cells express a semi-invariant TCR, which is composed of an invariant TCR a chain that pairs with a limited number of TCR b chains to form their cognate heterodimeric ab TCR. iNKT cell development follows an instructive mode, whereby the formation and expression of the semi-invariant TCR constitutes the essential first step of lineage determination.
In a large mutagenesis screen aimed at identifying critical components of the genetic network underlying T cell development, researchers at the Max Planck Institue of Immunobiology and Epigenetics identified a hitherto unrecognised aspect of TCR biology, namely the context-specific splicing of primary transcripts encoding the cognate TCR alpha chain gene.
mRNA template production for a specific receptor component
Primary transcripts of genes, so-called pre-mRNAs, are the first products to be produced when DNA is transcribed into RNA. In addition to sections that actually encode for proteins (exons), they still contain non-coding sections (introns). Accordingly, pre-mRNAs must undergo precise processing, known as splicing, to cut out intron sequences and produce mature mRNAs whose information can then be translated into functional proteins.
The splicing of pre-mRNAs depends on trans-acting factors, collectively referred to as the spliceosome, and cis-acting sequence motifs in the primary RNA transcript that demarcate the beginnings and ends of a specific type of noncoding segments that separate the coding regions. Primary transcripts arising from antigen receptor genes are no exception. TCR alpha genes also contain several intronic sequences that must be removed during the maturation process. One intron occurs in the variable (V) element, and another separates the J element from the first part of the so-called constant region. In the TCR a locus, which spans several tens of thousands of base pairs, this intron is very large, whereas all other introns quite small.
Transport protein controls the splicing process of immune cell primary transcripts
Owing to the complex, and often tissue-specific nature of the pre-mRNA splicing process, a large number of auxiliary splice regulators are employed to act in concert with the core spliceosome machinery. Many of these splice regulators are delivered to the nucleoplasm by dedicated protein transporters of the b-karyopherin family, such as Transportin 3 (Tnpo3).
Researchers in the Department of Developmental Immunology have identified an unexpectedly specific role of Tnpo3 in the pre-mRNA splicing process of the canonical TCRa chain of iNKT cells. In the absence of functional Tnpo3, the splicing process fails, and thus iNKT cells do not develop. This result is remarkable for two reasons, explains Thomas Boehm, the lead author of the study. “First, it is remarkable that the development (rather than differentiation) of an entire sub-lineage of T cells is absolutely dependent on just on factor in the complex hierarchy of splicing factors. Second, the fact that the splicing of the iNKT-type TCR alpha pre-mRNA is so specifically regulated and sparing others is an entirely new aspect of T cell biology.”
The study published in Nature Communications is another piece to the puzzle pursued in the Department of Developmental Immunology. It aims to identify the molecular basis of T cell development and at reconstructing the evolutionary trajectory of adaptive immune functions in vertebrates. “After this study in mice, we now know that the requirement of Tnpo3 for T cell development is evolutionarily conserved, as a Tnpo3 mutation in zebrafish, a representative of teleosts that have separated from the lineage giving rise to mammals, impairs T cell development as well,” explains project leader Norimasa Iwanami, who now works at Utsunomiya University in Japan.
The elucidation of the mechanisms and origins of dysfunction of key immune cells in different species promises not only to improve our understanding of the mammalian immune system, but could also be a source of inspiration for new medical diagnoses or therapeutic approaches.
TB/MR












